KRYSTAL-1 and KRYSTAL-12 EFFICACY
REGISTRATIONAL PHASE 2 COHORT1
THE ACCELERATED APPROVAL FOR KRAZATI® WAS BASED ON A ROBUST RESPONSE TO A RELENTLESS DISEASE2


58% HAD A RESPONSE DURATION OF ≥6 MONTHS2
- 42% of patients (n=112) achieved a PR, and 0.9% of patients achieved a CR3
- Median time to response was 1.4 months (range: 0.9–7.2)3
- At data cutoff, treatment was ongoing in 50% (24/48) of patients who experienced a response1,4
*Tumor response was assessed by BICR. Phase 2 data cutoff: October 15, 2021 (median follow-up: 12.9 months).3
BICR=blinded independent central review; CI=confidence interval; CR=complete response; mDOR=median duration of response; NSCLC=non-small cell lung cancer; ORR=objective response rate; PR=partial response.
References:
- Spira AI, Riely GJ, Gadgeel SM, et al. KRYSTAL-1: activity and safety of adagrasib (MRTX849) in patients with advanced/metastatic non-small cell lung cancer (NSCLC) harboring KRASG12C mutation. Oral presentation at ASCO 2022. Abstract 9002.
- KRAZATI®. Prescribing information. Princeton, NJ. Mirati Therapeutics, Inc., a Bristol Myers Squibb company; 2024.
- Jänne PA, Riely GJ, Gadgeel SM, et al. Adagrasib in non-small-cell lung cancer harboring a KRASG12C mutation. N Engl J Med. 2022;387(2):120-131.
- Data on file, Mirati Therapeutics.
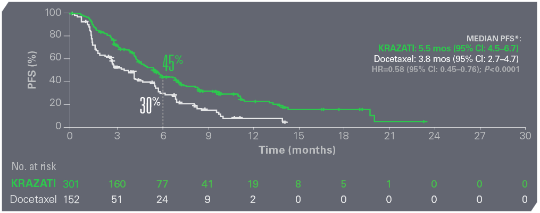
KRAZATI® DELIVERED SUPERIOR PFS AND ORR VS DOCETAXEL1
KRAZATI REDUCED THE RISK OF DISEASE PROGRESSION OR DEATH BY 42% VS DOCETAXEL
PFS WITH KRAZATI VS DOCETAXEL1
ORR WITH KRAZATI VS DOCETAXEL

Median follow-up time was 7.2 months.1 Data cutoff: December 31, 2023.1
*Per BICR (RECIST v1.1).1
BICR=blinded independent central review; CI=confidence interval; HR=hazard ratio; PFS=progression-free survival; ORR=objective response rate; RECIST=Response Evaluation Criteria in Solid Tumors.
Reference:
- Mok TSK, Yao W, Duruisseaux M, et al. KRYSTAL-12: phase 3 study of adagrasib versus docetaxel in patients with previously treated locally advanced or metastatic non-small cell lung cancer (NSCLC) harboring a KRASG12C mutation. Oral presentation at ASCO 2024. Abstract LBA8509.


