SAFETY OF KRAZATI
KRAZATI ADVERSE REACTIONS (ARs)1
ARs (≥20%) in Patients With KRAS G12C-Mutated NSCLC Who Received KRAZATI in KRYSTAL-11,2
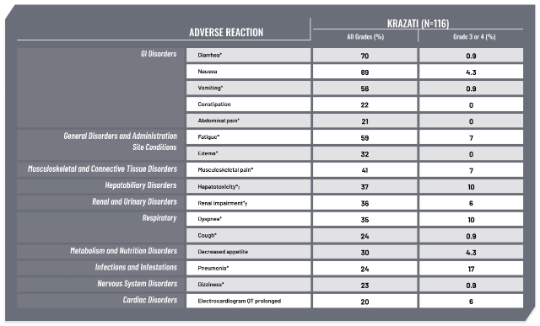
In KRYSTAL-1, 116 patients with KRAS G12C–mutated advanced NSCLC received KRAZATI 600 mg orally BID.1
Serious adverse reactions in ≥2% of patients were pneumonia (17%), dyspnea (9%), renal impairment (8%), sepsis (5%), hypoxia (4.3%), pleural effusion (4.3%), respiratory failure (4.3%), anemia (3.4%), cardiac failure (3.4%), hyponatremia (3.4%), hypotension (3.4%), muscular weakness (3.4%), pyrexia (3.4%), dehydration (2.6%), diarrhea (2.6%), mental status changes (2.6%), pulmonary embolism (2.6%), and pulmonary hemorrhage (2.6%). Fatal adverse reactions occurred in 11% of patients who received adagrasib due to pneumonia (3.4%), respiratory failure (1.7%), sudden death (1.7%), cardiac failure (0.9%), cerebrovascular accident (0.9%), mental status change (0.9%), pulmonary embolism (0.9%), and pulmonary hemorrhage (0.9%).1
*Grouped term.1
†Hepatotoxicity includes mixed liver injury, blood alkaline phosphatase increased, alanine aminotransferase increased, aspartate aminotransferase increased, liver function test increased, blood bilirubin increased, and bilirubin conjugated increased.1
‡Renal impairment includes acute kidney injury and increased blood creatinine.1
BID=twice-daily; GI=gastrointestinal; NSCLC=non-small cell lung cancer.
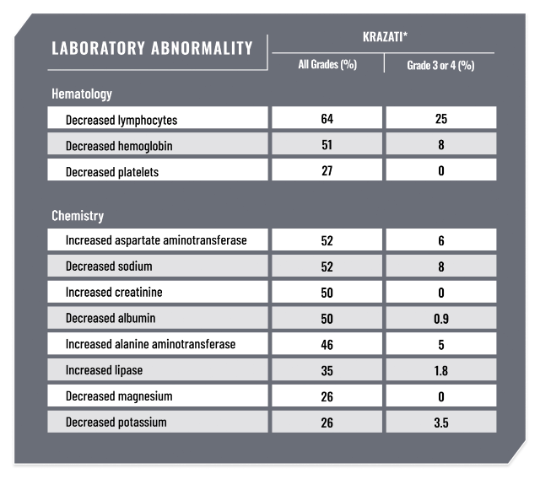
LABORATORY ABNORMALITIES1
Select Laboratory Abnormalities (Occurring in ≥25%) That Worsened From Baseline in Patients With KRAS G12C-Mutated NSCLC Who Received KRAZATI in KRYSTAL-1
NONE OF THE PATIENTS WHO RECEIVED PRIOR I-O <30 DAYS BEFORE KRAZATI TREATMENT EXPERIENCED GRADE ≥3 TREATMENT-RELATED HEPATOTOXICITY.3
There is no recommendation for a washout period after an I-O–based regimen in the KRAZATI Prescribing Information.
In KRYSTAL-1, patients underwent a 2-week washout period after treatment with most recent prior systemic therapy, including immunotherapy.
*Denominator used to calculate the rate varied from 106 to 113 based on the number of patients with a baseline value and at least one post-treatment value.1
ALT=alanine aminotransferase; AST=aspartate aminotransferase; I-O=immunotherapy.
USE OF COMMON GI CONCOMITANT MEDICATIONS IN KRYSTAL-1
Time to onset and resolution of select ARs4
Overall, 92% of patients with GI ARs (diarrhea, nausea, and vomiting) reported the initial event within the first 6 weeks.


Nausea, diarrhea, and vomiting were managed with dose reductions, interruptions, and/or use of supportive concomitant medications.

OF PATIENTS USED ANTIDIARRHEALS5

OF PATIENTS USED ANTIEMETICS/ANTINAUSEANTS5

OF PATIENTS USED PPIs5

OF PATIENTS USED H2-RECEPTOR ANTAGONISTS5
There were no clinically significant differences in the PK of adagrasib when used concomitantly with a PPI.1
FOR MORE INFORMATION ABOUT MONITORING AND MANAGEMENT, REFER TO THE KRAZATI PRESCRIBING INFORMATION AND THERAPY MANAGEMENT GUIDE.
*The range for the time to resolution after an initial occurrence was 0.1 to 50.1 weeks.4
†The range for the time to resolution after an initial occurrence was 0.3 to 15.1 weeks.4
GI=gastrointestinal; PK=pharmacokinetics; PPI=proton pump inhibitor.
KRAZATI DISCONTINUATION AND DOSAGE MODIFICATION RATES IN KRYSTAL-11


DOSE INTERRUPTIONS DUE TO AN AR
Adverse reactions (ARs) requiring dosage interruption in ≥2% of patients who received KRAZATI included nausea, hepatotoxicity, fatigue, vomiting, pneumonia, renal impairment, diarrhea, QTc interval prolongation, anemia, dyspnea, increased lipase, decreased appetite, dizziness, hyponatremia, muscular weakness, increased amylase, pneumonitis, sepsis, and decreased weight.

DOSE REDUCTIONS DUE TO AN AR
ARs which required dose reductions in ≥2% of patients who received KRAZATI included hepatotoxicity, fatigue, nausea, diarrhea, vomiting, and renal impairment.

DISCONTINUED DUE TO AN AR
ARs which resulted in permanent discontinuation of KRAZATI occurring in 2 patients each (1.7%) were pneumonia and pneumonitis, and occurring in 1 patient each (0.9%), were cerebrovascular accident, dyspnea, decreased ejection fraction, encephalitis, gastrointestinal obstruction, hemorrhage, hepatotoxicity, hypotension, muscular weakness, pulmonary embolism, pyrexia, respiratory failure, and sepsis.
Serious ARs were reported in 57% of patients receiving KRAZATI. Fatal ARs occurred in 11% of patients.
KRAZATI WARNINGS AND PRECAUTIONS1
GASTROINTESTINAL ADVERSE REACTIONS
KRAZATI can cause severe gastrointestinal adverse reactions.
In the pooled safety population, serious gastrointestinal adverse reactions observed were gastrointestinal bleeding in 3.8% including 0.8% Grade 3 or 4, gastrointestinal obstruction in 1.6% including 1.4% Grade 3 or 4, colitis in 0.5% including 0.3% Grade 3, ileus in 0.5%, and stenosis in 0.3%. In addition, nausea, diarrhea, or vomiting occurred in 89% of 366 patients, including 9% Grade 3. Nausea, diarrhea, or vomiting led to dosage interruption or dose reduction in 29% of patients and permanent discontinuation of KRAZATI in 0.3%.
Monitor and manage patients using supportive care, including antidiarrheals, antiemetics, or fluid replacement, as indicated. Withhold, reduce the dose, or permanently discontinue KRAZATI based on severity.
QTc INTERVAL PROLONGATION
KRAZATI can cause QTc interval prolongation, which can increase the risk of ventricular tachyarrhythmias (eg, torsades de pointes) or sudden death.
In the pooled safety population, 6% of 366 patients with at least one post-baseline electrocardiogram (ECG) assessment had an average QTc ≥501 ms and 11% of patients had an increase from baseline of QTc >60 msec. KRAZATI causes concentration-dependent increases in the QTc interval.
Avoid concomitant use of KRAZATI with other products with a known potential to prolong the QTc interval. Avoid use of KRAZATI in patients with congenital long QT syndrome and in patients with concurrent QTc prolongation.
Monitor ECGs and electrolytes prior to starting KRAZATI, during concomitant use, and as clinically indicated in patients with congestive heart failure, bradyarrhythmias, electrolyte abnormalities, and in patients who are unable to avoid concomitant medications that are known to prolong the QT interval. Withhold, reduce the dose, or permanently discontinue KRAZATI depending on severity.
FOR MORE INFORMATION ABOUT MONITORING AND MANAGEMENT, REFER TO THE KRAZATI PRESCRIBING INFORMATION AND THERAPY MANAGEMENT GUIDE.
HEPATOTOXICITY
KRAZATI can cause hepatotoxicity, which may lead to drug-induced liver injury and hepatitis.
In the pooled safety population of 366 patients, drug-induced liver injury was reported in 0.3% of patients, including 0.3% Grade 3. A total of 32% of patients who received KRAZATI had increased alanine aminotransferase (ALT)/increased aspartate aminotransferase (AST); 5% were Grade 3 and 0.5% were Grade 4. The median time to first onset of increased ALT/AST was 3 weeks (range: 0.1 to 48). Overall hepatotoxicity occurred in 37%, and 7% were Grade 3 or 4. Hepatotoxicity leading to dose interruption or reduction occurred in 12% of patients. KRAZATI was discontinued due to hepatotoxicity in 0.5% of patients.
Monitor liver laboratory tests (AST, ALT, alkaline phosphatase and total bilirubin) prior to the start of KRAZATI and monthly for 3 months or as clinically indicated, with more frequent testing in patients who develop transaminase elevations. Reduce the dose, withhold, or permanently discontinue KRAZATI based on severity.
INTERSTITIAL LUNG DISEASE/PNEUMONITIS
KRAZATI can cause interstitial lung disease (ILD)/pneumonitis, which can be fatal.
In the pooled safety population, ILD/pneumonitis occurred in 4.1% of patients, 1.4% were Grade 3 or 4, and one case was fatal. The median time to first onset for ILD/pneumonitis was 12 weeks (range: 5 to 31 weeks). KRAZATI was discontinued due to ILD/pneumonitis in 0.8% of patients.
Monitor patients for new or worsening respiratory symptoms indicative of ILD/pneumonitis (eg, dyspnea, cough, fever) during treatment with KRAZATI.
Withhold KRAZATI in patients with suspected ILD/pneumonitis and permanently discontinue KRAZATI if no other potential causes of ILD/pneumonitis are identified.
FOR MORE INFORMATION ABOUT MONITORING AND MANAGEMENT, REFER TO THE KRAZATI PRESCRIBING INFORMATION AND THERAPY MANAGEMENT GUIDE.
References:
- KRAZATI®. Prescribing information. Princeton, NJ. Mirati Therapeutics, Inc., a Bristol Myers Squibb company; 2024.
- Data on file, Mirati Therapeutics. 2022.
- Gadgeel SM, Jänne PA, Spira AI, et al. KRYSTAL-1: two-year follow-up of adagrasib (MRTX849) monotherapy in patients with advanced/metastatic KRASG12C-mutated NSCLC. Presented at World Conference on Lung Cancer; September 9-12, 2023; Singapore.
- Jänne PA, Riely GJ, Gadgeel SM, et al. Adagrasib in non-small-cell lung cancer harboring a KRASG12C mutation. N Engl J Med. 2022;387(2):120-131.
- Zhang J, Johnson M, Barve M, et al. Practical guidance for the management of adverse events in patients with KRASG12C-mutated non-small cell lung cancer receiving adagrasib. Oncologist. 2023;28(4):287-296.
CONSISTENT SAFETY PROFILE WITH KRYSTAL-1: NO NEW SAFETY SIGNALS
SAFETY SUMMARY IN KRYSTAL-121

AE=adverse event; SAE=serious adverse event; TEAE=treatment-emergent adverse event.
FOR MORE INFORMATION ABOUT MONITORING AND MANAGEMENT, REFER TO THE KRAZATI PRESCRIBING INFORMATION AND THERAPY MANAGEMENT GUIDE.
Reference:
- Data on file, Mirati Therapeutics.


