KRYSTAL-1 and KRYSTAL-12 STUDY DESIGNS
KRYSTAL-1 STUDY DESIGN1
Registrational study of efficacy and safety
The safety and efficacy of KRAZATI were evaluated in patients with KRAS G12C–mutated, locally advanced or metastatic NSCLC who received at least 1 prior systemic therapy in KRYSTAL-1, a multicenter, single-arm, open-label expansion cohort study. 116 patients were enrolled; 112 were evaluable.1

KEY INCLUSION CRITERIA
Locally advanced or metastatic NSCLC1
Confirmed KRAS G12C mutation determined in tumor tissue1
Previously treated with an I-O-based regimen1*
Stable brain metastases allowed2†

KRAZATI 600 mg BID
Until unacceptable toxicity or disease progression1




Key exclusion criteria included history of intestinal disease or major gastric surgery likely to alter absorption of medication or inability to swallow oral medications, active brain metastases, carcinomatous meningitis, and other active cancer.2
*Platinum-based regimen and an immune checkpoint inhibitor.1
†Patients were eligible if brain metastases were adequately treated and patients were neurologically stable ≥2 weeks prior to enrollment without the use of corticosteroids or were on a stable or decreasing dose of ≤10 mg daily prednisone (or equivalent).2
BID=twice-daily; DOR=duration of response; I-O=immunotherapy; NSCLC=non-small cell lung cancer; ORR=objective response rate.
BASELINE DEMOGRAPHICS1,2
There is no recommendation for a washout period after an I-O-based regimen in the KRAZATI Prescribing Information.
In KRYSTAL-1, patients underwent a 2-week washout period after treatment with most recent prior systemic therapy, including immunotherapy.
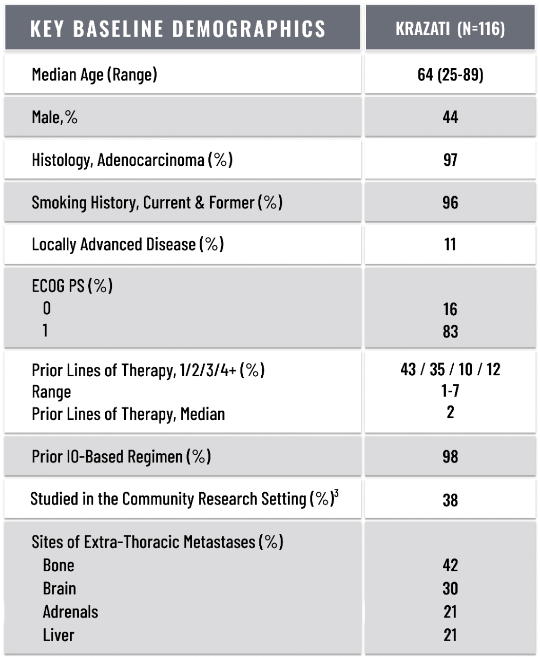
ECOG PS=Eastern Cooperative Oncology Group Performance Status.
References:
- KRAZATI®. Prescribing information. Princeton, NJ. Mirati Therapeutics, Inc., a Bristol Myers Squibb company; 2024.
- Jänne PA, Riely GJ, Gadgeel SM, et al. Adagrasib in non-small-cell lung cancer harboring a KRASG12C mutation. N Engl J Med. 2022;387(2):120-131.
- Data on file. Cohort A Academic or Community. Princeton, NJ: Mirati Therapeutics, Inc., a Bristol Myers Squibb company; 2024.
KRYSTAL-12 STUDY DESIGN1
Phase 3 study of efficacy and safety

KEY INCLUSION CRITERIA1
Locally advanced or metastatic NSCLC with KRAS G12C mutation
Prior treatment with platinum-based chemotherapy and
anti–PD-(L)1 therapyECOG PS 0–1
Stable brain metastases allowed






Key exclusion criteria included prior treatment with a
KRAS G12C inhibitor and/or active brain metastases.2
*Crossover from docetaxel to KRAZATI was allowed in cases where disease progression per RECIST v1.1 was confirmed by real-time BICR. Other crossover criteria: ECOG PS
0–2, recovery from DOCE-related AEs to grade 1 or baseline (except peripheral neuropathy and alopecia for which grade 2 is acceptable).1
†Per BICR (RECIST v1.1)1
AE=adverse event; BICR=blinded independent central review; BID=twice-daily; DOCE=docetaxel; DOR=duration of response; ECOG PS=Eastern Cooperative Oncology Group Performance Status; IV=intravenous; NSCLC=non-small cell lung cancer; ORR=objective response rate; PD-L1=programmed death-ligand 1; PFS=progression-free survival; PO=orally; Q3W=every 3 weeks; RECIST=Response Evaluation Criteria in Solid Tumors.
BASELINE DEMOGRAPHICS1
In KRYSTAL-12, 100% of patients previously received an I-O–based regimen1

I-O=immuno-oncology.
References:
- Mok TSK, Yao W, Duruisseaux M, et al. KRYSTAL-12: phase 3 study of adagrasib versus docetaxel in patients with previously treated locally advanced or metastatic non-small cell lung cancer (NSCLC) harboring a KRASG12C mutation. Oral presentation at ASCO 2024. Abstract LBA8509.
- Clinicaltrials.gov. NCT04685135. Accessed July 3, 2025.


